Hylartin V
$75.00
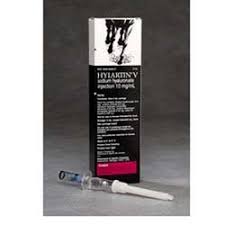
Hylartin V A sterile pyrogen-free solution of a highly purified, specific fraction of the sodium salt of hyaluronic acid extracted from rooster combs.
Hylartin V A sterile pyrogen-free solution of a highly purified, specific fraction of the sodium salt of hyaluronic acid extracted from rooster combs.
Description
Hylartin V A sterile pyrogen-free solution of a highly purified, specific fraction of the sodium salt of hyaluronic acid extracted from rooster combs. Indicated in the treatment of joint dysfunction in horses due to non-infectious synovitis associated with equine osteoarthritis.
- Sodium hyaluronate injection.
- Supplied in disposable glass syringes, contains 20mg (10mg/ml) of sodium hyaluronate in 2ml.
- Given to horses intra-articularly in small and medium size joints (carpal, fetlock). In the treatment of largers joints (hock), the dosage is 4ml (40mg). The treatment may be repeated at weekly intervals for a total of 3 treatments.
Undisputed Heavyweight Champion for Equine Lameness
HYLARTIN® V Injection (sodium hyaluronate) helps horses’ joints heal faster. It lubricates joint tissues, reduces friction, alleviates pain and improves joint action. HYLARTIN V is the heavyweight joint therapy champion, preferred by professionals who demand the most performance and protection available.
Hylartin V
This page contains information on Hylartin V for veterinary use.
The information provided typically includes the following:
- Hylartin V Indications
- Warnings and cautions for Hylartin V
- Direction and dosage information for Hylartin V
Hylartin V
This treatment applies to the following species:
Company: Zoetissodium hyaluronate injection
10 mg/mL
Product Information
Hylartin V Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Description
HYLARTIN® V is a sterile pyrogen-free solution of a highly purified, specific fraction of the sodium salt of hyaluronic acid extracted from rooster combs. HYLARTIN® V is supplied in disposable glass syringes, each of which contains 20 mg (10 mg/mL) of sodium hyaluronate in 2.0 mL physiological sodium chloride-phosphate buffer with a pH of 7.0-7.5.
CHEMISTRY:
Sodium hyaluronate is a high molecular weight polymer made up of repeating disaccharide units of N-acetylglucosamine and sodium glucuronate linked by beta 1-3 and beta 1-4 glycosidic bonds. HYLARTIN® V contains only traces of protein.
Pharmacology
Sodium hyaluronate is a natural, physiological substance which occurs extracellularly in connective tissue in both animals and man and is chemically identical in different species. High concentrations (>0.2 mg/mL) of hyaluronate are found in the synovial fluid, the vitreous of the eye and the umbilical cord.
Sodium hyaluronate is a normal component of connective tissue matrix and it is injected therapeutically only in compartments where it constitutes a normal component, specifically the joint cavity.
ANIMAL SAFETY:
Acute, sub-acute and chronic toxicity studies in mice, rats, rabbits, dogs, monkeys and horses have not demonstrated any significant adverse reactions or sensitization.
In an acute toxicity study in horses, HYLARTIN® V was injected intra-articularly at dosages corresponding to five times the recommended dose per animal (200 mg total). In a sub-acute study, horses were injected intra-articularly with the recommended dose per joint (20 mg) at weekly intervals for nine weeks. The results of both investigations showed that hematological and blood chemistry values remained within normal ranges. In mice, the intravenous LD100 was found to be of the order of 50 mg/kg body weight.
There is always a potential immunological risk with repeated parenteral administration of biological material. However, as shown by Richter (1974), sodium hyaluronate, of both human and avian origin, did not produce any antibodies after repeated immunization, nor did intense stimulation of the immunization process by coupling of protein to the hyaluronate and simultaneous administration of Freund’s adjuvant give rise to antibodies.
CLINICAL STUDIES:
Clinical field trials with thoroughbred and standardbred race horses were undertaken at four separate clinics. A total of 252 joints were injected with HYLARTIN® V in these investigations. In one study, only horses which were conventional treatment failures were included and the overall improvement rate following HYLARTIN® V treatment approached 90 percent. In the other studies, the improvement rate surpassed this figure.
In another case, electrogoniometry







Reviews
There are no reviews yet.